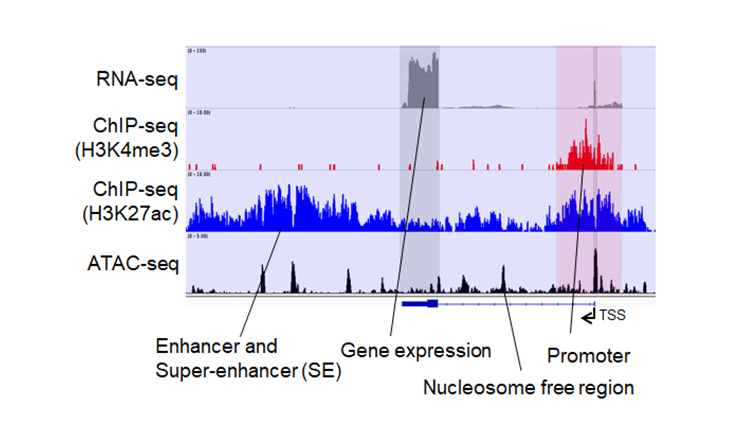
【Research Outline】
Pharmacology research aimed at elucidating the biological basis that controls stress and aging of brain function and resilience toward therapeutic drug development
Pharmacology is a discipline that elucidates the molecular mechanism responsible for the interaction between drugs and living organisms and the pathophysiology of diseases and contributes to drug treatment and drug discovery. We study stress and brain aging related to mental and physical health and illnesses from the pharmacology viewpoint.
Stress due to adverse environments and demanding conditions causes diverse effects on mental and physical functions. For example, acute and controllable stress promotes adaptive responses to cope with stress, increasing habituation and resilience to stress. On the other hand, chronic and uncontrollable stress induces depression, elevated anxiety, and cognitive dysfunction, increasing the risks for mental and physical illnesses, such as depression. In addition, chronic and uncontrollable stress does not necessarily cause depression and elevated anxiety in all individuals, and there is considerable individual variability in stress susceptibility, suggesting the presence of stress resilience. However, stress and resilience mechanisms remain poorly understood, and therapeutic drug development targeting stress is not established.
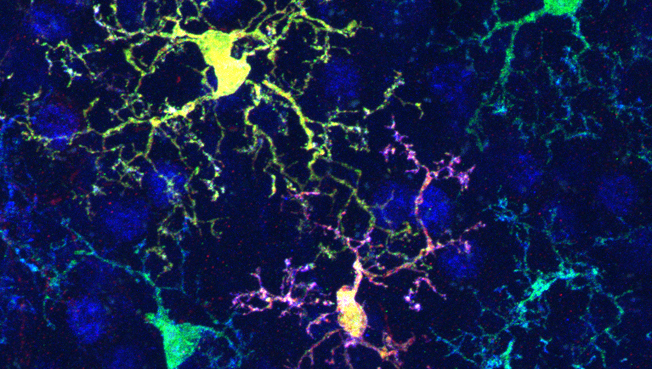
To address this issue, we have been conducting biological studies using mouse stress models, such as social defeat stress. We demonstrated that the impacts of stress on brain structures and functions and behaviors vary depending on stress conditions. Thus, acute stress causes dendrite growth of neurons in the medial prefrontal cortex via the neurotransmitter molecule dopamine, augmenting stress resilience. By contrast, chronic stress induces emotional disturbances along with dendrite shrinkage of neurons in the medial prefrontal cortex via inflammatory molecules derived from microglia, inflammation-related cells in the brain. In addition, it is becoming clear that chronic stress causes leukocyte mobilization from the bone marrow and infiltration into the brain, contributing to emotional disturbances.
Thus, the biological basis of stress-induced emotional disturbances is molecular and cellular changes of neurons and glia in the brain, cooperating with leukocytes that infiltrate from the periphery into the brain. These interactions alter the activity patterns of local and distributed neuronal networks, leading to emotional disturbances.
Besides, brain aging is also associated with motivation and cognitive decline. Neuroinflammation derived from dendritic shrinkage of neurons and microglial activation is implicated in brain aging. Individual variability in brain aging suggests the presence of resilience to it. However, what and how brain aging occurs remains elusive.
In this laboratory, we aim to elucidate the biological basis that underlies stress-induced mental and physical alterations, aging of brain function, and resilience to stress and aging toward developing innovative therapeutic drugs for mental and physical illnesses such as depression.
【Main Research Themes】
Elucidation of stress-induced cellular changes in the brain and their significance
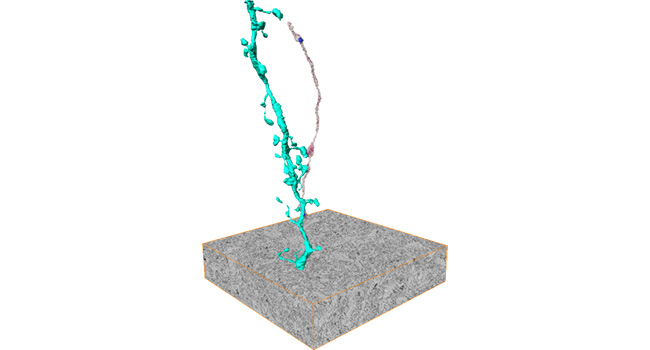
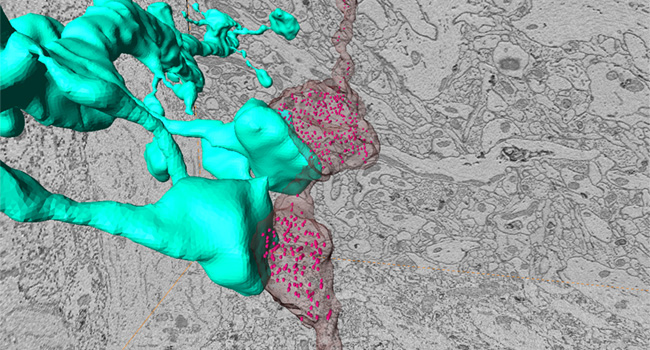
Stress-induced changes in structures and functions of neurons and glia in the brain are crucial for cognitive and emotional disturbances and mental illnesses. However, the changes in subcellular organelles and their mechanisms remain unknown. We aim to elucidate stress-induced cellular changes in the brain and their significance using histological analyses with three-dimensional electron microscopy and expansion microscopy, multi-omics analyses, brain region- and cell type-specific molecular manipulations with recombinant viruses, and mouse behavior experiments, partly through collaborations.
Elucidation of stress-induced transcriptional and epigenetic regulations in the brain and their significance
Since neuronal and glial responses to stress are altered with chronic stress, these changes may involve transcriptional and epigenetic regulations, which remain unknown. We aim to elucidate stress-induced transcriptional and epigenetic regulations in respective cell types in the brain and their significance using brain region- and cell type-specific gene expression, transcriptional and epigenetic analyses, single-cell omics analyses with cell sorting from respective brain regions, brain region- and cell type-specific molecular manipulations with recombinant viruses, and mouse behavior experiments, partly through collaborations.
Elucidation of neural circuits and their information coding that underlie stress susceptibility and resilience
Stress-induced molecular and cellular changes in the brain lead to emotional disturbances through changes in the activity patterns of neural circuits composed of multiple brain regions. The importance of the medial prefrontal cortex, the nucleus accumbens, and the monoaminergic projections to these brain regions has been established. However, the entire neural circuits that control stress susceptibility and resilience and their information coding remain unknown. We aim to elucidate neural circuits that control stress sensitivity and resilience and the activity patterns of these neuronal circuits by whole-brain neural activity mapping, electrophysiological recording, and brain region- and neuronal projection-specific manipulation of neuronal activities, partly through collaborations.
Elucidation of stress-induced inflammatory responses and their significance
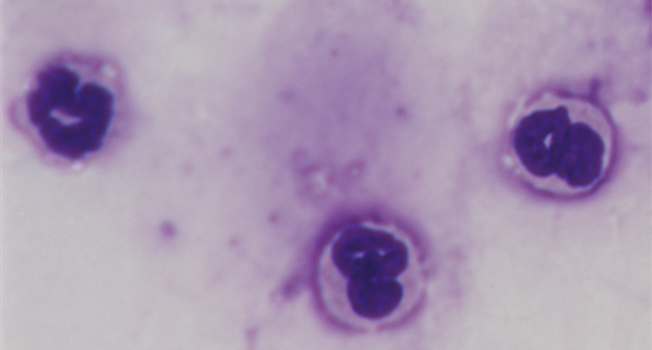
We and others demonstrated crucial roles of neuroinflammation mediated by various inflammation-related molecules such as bioactive lipids and cytokines for stress-induced emotional disturbances and mental illnesses. Stress also induces systemic inflammation manifested by the production of inflammation-related molecules and the mobilization of leukocytes in the blood, which may contribute to functional changes in the brain and peripheral organs and their interplay. We aim to elucidate stress-induced inflammatory responses in the brain and periphery and their significance using comprehensive analyses of bioactive lipids and cytokines, flow cytometry analyses of leukocytes, single-cell omics analyses, tissue- and cell type-specific genetically modified mice, and mouse behavioral experiments, partly through collaborations.
Elucidation of the biological basis of brain aging
We will evaluate altered brain functions, such as motivation and cognitive decline, in aged mice in multiple dimensions using various cognitive tasks, including stimulus discrimination task and attention set shift task, besides conventional emotional tests. We will then elucidate the mechanisms responsible for respective cognitive and emotional alterations associated with aging using multi-omics analyses, histological analyses, and molecular and neuronal activity manipulations, partly through collaborations.
Go to top【Outlook】
We will promote basic research using mouse stress models and aged mice, and if necessary, conduct clinical research using specimens of healthy subjects and patients, partly through collaborations. We aim to biologically address the fundamental problems about stress, brain aging, and resilience and develop innovative therapeutic drugs that overcome mental and physical illnesses, including neuropsychiatric disorders.
- How is stress generated, amplified, and maintained in the brain?
- How are the susceptibility and resilience to stress and aging controlled?
- How does individual variability in stress and brain aging occur?
- How are neural and bodily changes with stress and aging related?
- How are stress and aging related to mental and physical illnesses?
- How can mental and physical dysfunctions with stress and aging be restored?
【Methods】(including those through collaborations)
Mouse behavior experiments (P1A/P2A), generation of genetically modified mice, brain stereotactic surgery, generation of mice inoculated with recombinant viruses, implantation with a cannula for intracerebral administration, implantation with osmotic pumps, optogenetic/chemogenetic manipulation, in vivo imaging of neuronal activities, cell sorting, flow cytometry, immunohistochemistry, laser confocal microscopic observation, three-dimensional electron microscopic analysis, expansion microscopic analysis, automated image analysis, comprehensive gene expression analysis (RNA-seq), comprehensive epigenome analysis (chromatin immunoprecipitation and ATAC sequencing), comprehensive lipid analysis (LC-MS/MS), comprehensive cytokine analysis, primary cultured neurons and glia, cell transfection, biochemical slice experiments, HPLC-ECD, plasmid construction, generation of recombinant viruses, quantitative RT-PCR
Go to top【References】
- Furuyashiki T, Narumiya S. Stress responses: the contribution of prostaglandin E2 and its receptors. Nature Reviews Endocrinology 7, 163-175 (2011).PubMed
- Tanaka K et al. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. Journal of Neuroscience 32, 4319-4329 (2012).PubMed
- Shinohara R et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Molecular Psychiatry 23, 1717-1730 (2018).PubMed
- Nie X et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron 99, 464-479 (2018).PubMed
- Furuyashiki T et al. Roles of multiple lipid mediators in stress and depression. International Immunology 31, 579-587 (2019).PubMed
- Furuyashiki T and Kitaoka S. Neural mechanisms underlying adaptive and maladaptive consequences of stress: Roles of dopaminergic and inflammatory responses. Psychiatry and Clinical Neurosciences 73, 669-675 (2019).PubMed
- Nie X et al. Roles of Toll-like receptor 2/4, monoacylglycerol lipase, and cyclooxygenase in social defeat stress-induced prostaglandin E2 synthesis in the brain and their behavioral relevance. Scientific Reports 9, 16670 (2019).PubMed
- Ishikawa Y et al. Repeated social defeat stress induces neutrophil mobilization in mice: maintenance after cessation of stress and strain-dependent difference in response. British Journal of Pharmacology 178, 827-844 (2021).PubMed
- Akiyama S et al. Chronic social defeat stress increases the amounts of 12-lipoxygenase lipid metabolites in the nucleus accumbens of stress-resilient mice. Scientific Reports 12, 11385 (2022).PubMed
- Kitaoka S et al. Repeated social defeat stress induces HMGB1 nuclear export in prefrontal neurons, leading to social avoidance in mice. Cells 12, 1789 (2023).PubMed
- Horikawa I et al. Chronic stress alters lipid mediator profiles associated with immune-related gene expressions and cell compositions in mouse bone marrow and spleen. Journal of Pharmacological Sciences 154, 279-293 (2024).PubMed
- Okuda Y et al. The activation of the piriform cortex to lateral septum pathway during chronic social defeat stress is crucial for the induction of behavioral disturbance in mice. Neuropsychopharmacology 50, 828-840 (2025).PubMed
【Recruitment】
Our laboratory is recruiting graduate and undergraduate students interested in biological studies about stress, brain aging, and resilience. For those who are interested in these studies, please contact us with the information below.
Tomoyuki Furuyashiki (Professor, Division of Pharmacology, Graduate School of Medicine, Kobe University)
TEL 078-382-5440;
FAX 078-382-5459;
tfuruya[at]med.kobe-u.ac.jp
([at] should be read as @)
Go to top
【Principal Investigator】
FURUYASHIKI Tomoyuki
Professor, Division of Pharmacology, Graduate School of Medicine, Kobe University
- Apr 1991 – Mar 1997 Medical student in Kyoto University Faculty of Medicine, Kyoto, Japan
- Apr 1997 – Mar 2001 Graduate student in Department of Pharmacology, Kyoto University Graduate School of Medicine, Japan
- Apr 2001 – Mar 2003 Postdoctoral fellow of the Japan Society for the Promotion of Science
- Apr 2003 – Aug 2007 Assistant professor in Kyoto University Graduate School of Medicine
- Sep 2004 – Mar 2008 Associate Research Scientist in Department of Psychological and Brain Sciences, Johns Hopkins University
- Apr 2008 – Oct 2012 Assistant professor in Kyoto University Graduate School of Medicine
- Nov 2012 – Apr 2014 Associate professor in Kyoto University Graduate School of Medicine
- May 2014 – Present Professor and Chair in Division of Pharmacology, Kobe University Graduate School of Medicine
- March 2012 The Young Investigator Award from the Japanese Pharmacological Society
- November 2015 The Best President’s Award from the Astellas Foundation for Research on Metabolic Disorders
- May 2024 CINP (The International College of Neuropsychopharmacology) Sumitomo Pharma Brain Health Basic Research Award
【構成員】
| Name | Job Title | Phone | Email address
([at] should be read as @) |
|---|---|---|---|
| FURUYASHIKI Tomoyuki | Professor | 81-78-382-5440 | tfuruya[at]med.kobe-u.ac.jp |
| SHINOHARA Ryota | Associate Professor | 81-78-382-5701 | rshino[at]med.kobe-u.ac.jp |
| TANIGUCHI Masayuki | Assistant Professor | 81-78-382-5441 | m-taniguchi[at]port.kobe-u.ac.jp |
| YAMAGUCHI Yuta | Assistant Professor | 81-78-382-5441 | yyuta[at]med.kobe-u.ac.jp |
| KOMAKI Ryohei | Assistant Professor | 81-78-382-5442 | ryouheik[at]med.kobe-u.ac.jp |
| OKUDA Yuki | Assistant Professor |
81-78-382-5701 | okudayuki[at]dolphin.kobe-u.ac.jp |
| IWAMURA Hiroko | Technical Staff (Technical) |
81-78-382-5442 | ー |
| ANDO Akemi | Administrative Staff | 81-78-382-5443 | ー |
| MATSUSHITA Kazutoshi | PhD Student (since 2018) |
ー | ー |
| ZHU Qing | PhD Student (since 2021) |
ー | ー |
| OTA Kohei | PhD Student (since 2023) |
ー | ー |
| TIAN Bowen | PhD Student (since 2023) |
ー | ー |
| QIU Wenran | PhD Student (since 2024) |
ー | ー |
| LI Dongrui | PhD Student (since 2024) |
ー | ー |
| ZHU Yunhui | PhD Student (since 2024) |
ー | ー |
| CHEN Guowei | PhD Student (since 2024) |
ー | ー |
| MUNAWAROH Fauziyatul | PhD Student (since 2024) |
ー | ー |
| YAMAMOTO Marina | PhD Student (since 2025) |
ー | ー |
| KAWASE Nozomu | PhD Student (since 2025) |
ー | ー |
| OTA Rinka | Master's Student (since 2024) |
ー | ー |
| TAKEUCHI Koji | Master's Student (since 2024) |
ー | ー |
| JIN Boxiang | Master's Student (since 2025) |
ー | ー |
| KITAOKA Chisato | Master's Student (since 2025) |
ー | ー |
| Medical students | ー | ||
【Access】
Tomoyuki Furuyashiki (Professor, Division of Pharmacology, Graduate School of Medicine, Kobe University)
TEL 078-382-5440;
FAX 078-382-5459;
tfuruya[at]med.kobe-u.ac.jp
([at] should be read as @)
Our laboratory is located on the east side of the 4th floor of Research Building B in the Kusunoki Campus of Kobe University.
Please refer to the following URL for access to the Graduate School of Medicine, Kobe University, and the location of Research Building B.
https://www.med.kobe-u.ac.jp/access/