GALLERY
@ @Click a thumbnail to expand the image or play the video.|
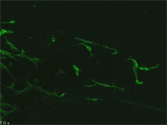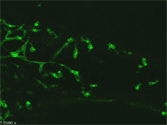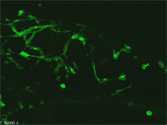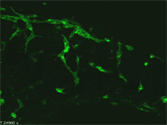
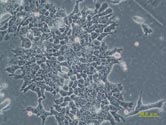
EB3/5 cells |
|
|
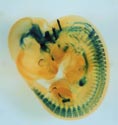
X-gal staining of a mouse embryo (E11.5) expressing tauLacZ under the Gfr¿1 promoter. GFR¿1 is a GPI-anchored cell surface receptor that binds to GDNF with high affinity. Signals are detected in the brain, enteric nervous system, kidneys and peripheral sensory ganglia. |
|
|
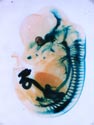
X-gal staining of a mouse embryo (E12.5) expressing tauLacZ under the Ret promoter. RET is a receptor tyrosine kinase that is activated by GDNF family ligands bound to GFR¿ receptors. Signals are detected in the brain, peripheral autonomic and sensory ganglia, and kidneys. |
|
|
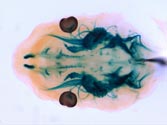
Top view of the head of a Ret tauLacZ/+ embryo (E12.5) subjected to X-gal staining. Signals are detected in the cranial sensory (trigeminal) and parasympathetic (sphenopalatine and otic) ganglia and in the brain (midbrain dopaminergic neurons etc.). |
|
|
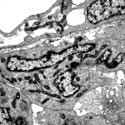
Transmission electron microscopy of enteric neural crest-derived cells in a mouse model of Hirschsprung disease (intestinal aganglionosis). In this mouse model, ENCCs are lost due to caspase-independent non-apoptotic cell death, which leads to the absence of enteric ganglia in the distal colon. These data reveal the crucial role of cell death in the etiology of Hirschsprung disease. |
|
|
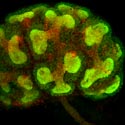
Immunohistochemical detection of GFP (green) and E-cadherin (red) in a developing kidney of GFR¿1-GFP knockin mice. GFR¿1 is expressed in the ureteric bud and its surrounding metanephric blastema. E-cadherin is detected in cell-cell junctions of the ureteric bud. |
|
|
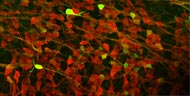
Genetic cell tracing using the Cre-loxP system enables visualization of the morphology of single neurons and their processes (green) against the whole neuronal population (PGP9.5 staining: red) in the developing enteric nervous system. | |
|
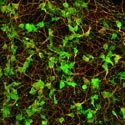
Neural progenitors in the peripheral ganglia can be selectively expanded in vitro by a neurosphere culture method. These progenitors can differentiate into neurons and glia. This photograph shows the robust neuronal differentiation of neurosphere cells isolated from the parasympathetic ganglia of RET-EGFP knockin embryos. RET-expressing cells (green) co-express PGP9.5, a pan-neuronal marker (red). |
|
|
Immunostaining of differentiated neurosphere cells generated from mouse sympathetic ganglia. Neurons are recognized by anti-class III À tubulin antibody (green; cell bodies and neurites) and anti-Phox2b antibody (blue; nuclei). The nuclei of glial cells are visualized by anti-Sox10 antibody (red). |
|
|
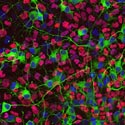
Immunofluorescent detection of Phox2b (magenta) and PGP9.5 (green) in a whole-mount preparation of mouse embryonic gut (E13.5). Phox2b is expressed in both undifferentiated neural progenitors and neurons. Note that, at this developmental stage, only a subpopulation of Phox2b-positive cells express PGP9.5, a pan-neuronal marker. |
|
|
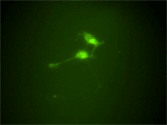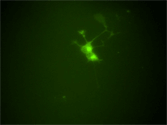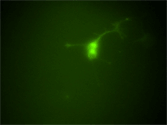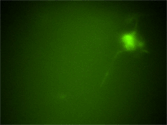
Visualization of the RET tyrosine kinase in living enteric neurons. These neurons were isolated from mice engineered to express a RET-EGFP fusion protein under the endogenous Ret promoter. Unlike Ret-deficient mice, which die at birth due to intestinal aganglionosis and kidney agenesis, mice homozygous for the RET-EGFP allele display no abnormalities in RET-dependent organogenesis. RET-EGFP mice serve as a valuable platform for examining the localization and trafficking of RET in living cells and tissues. |
|
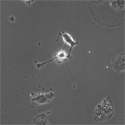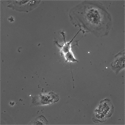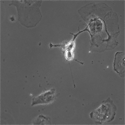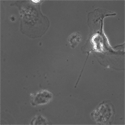
|
Phase contrast view of the same movie as above. Note the highly dynamic morphological changes of the growth cone after its contact with a mesenchymal cell of the gut. |
|
|
Time-lapse imaging analysis of enteric neural crest-derived cells (ENCCs) in the hindgut of RET-EGFP homozygous mice (E12.5). This movie was taken by examining a gut organ culture using a laser confocal microscope. In migrating ENCCs, RET-EGFP signals were more prominent in the frontal region in the direction of their migration, suggesting that polarized localization of RET is crucial for cell migration. | |





_PGP9(G)_t.jpg)