Research interest
Our lab investigates the molecular mechanisms of sphingosine 1-phosphate (S1P) signaling that link animal phisiology and disease.
S1P, a phosphorylated product of sphingosine catalyzed by sphingosine kinase (SphK), has been implicated as an important lipid mediator acting both inside and outside the cells. Extracellular S1P binds to members of GTP-binding protein (G-protein)-coupled S1P receptor family (S1P1-5), triggering diverse cellular effects including angiogenesis, cardiac development, immunity, cell motility, neurite extension, and neurotransmitter secretion. On the other hand, S1P has been shown to function intracellularly, mediating mobilization of cellular calcium, cell growth, and suppression of apoptosis.
We try to understand the role of SphK-S1P-S1P receptor system on phisiological phenomenon of cell and living organisms.
Topics
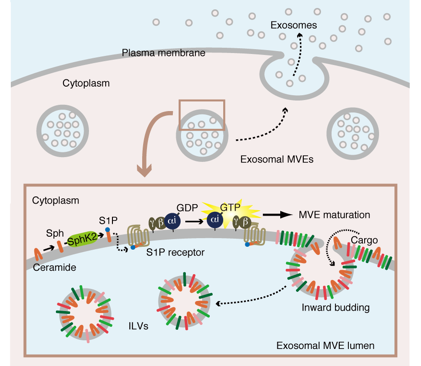
Sphingosine 1-phosphate (S1P) triggers the sorting of cargo protein into exosome
Exosomes are small membrane bound vesicles with a diameter of 50–100nm released from a variety of cells important for erythrocyte maturation, cell-to-cell communication, antigen presentation, prion diseases, tumour metastasis and Alzheimer’s disease. However, the precise mechanisms underlying cargo sorting into exosomes were entirely unknown.
Recently we found the SphK-S1P-S1P receptor system plays a critical role in cargo protein sorting into exosome. In this report there are two important findings.
1) Gi-coupled S1P receptors on multivesicular endosomes (MVEs) are constitutively activated through a constant supply of S1P via autocrine activation within organelles.
2) The continuous activation of Gi-coupled S1P receptors on MVEs is essential for cargo protein sorting into exosome.
Kajimoto, T., Okada, T., Miya, S., Zhang, L., Nakamura, SI. Ongoing activation of sphingosine 1-phosphate receptors
mediates maturation of exosomal multivesicular endosomes. Nat. Commun. 4:2712 doi: 10.1038/ncomms3712 (2013)
During MVE maturation the level of ceramide increases at the expense of sphingomyelin, which is rapidly degraded by various sphingomyelinases. Ceramide is sequentially metabolized to S1P by SphK2 via sphingosine. Upon stimulation by S1P, the activation of Gi-coupled S1P receptor triggers the dissociation of Gi subunit Gαi and βγ subunit. This “ongoing activation” of Gi-coupled S1P receptor on the MVE is essential for cargo protein sorting into exosomes.
Suppression of cargo protein (CD63) sorting into ILVs of MVEs by an SphK inhibitor, HACPT. HeLa cells expressing both CD63-GFP and FLAG-Rab5 (Q79L) were pretreated with DMSO vehicle or 5 μM HACPT for 24 h. Cell medium was changed in Hanks' balanced salt solution (HBSS) containing 1 mM CaCl2 and 5 mM HEPES and then treated with 10 μM nocodazole for 30min. CD63-GFP fluorescence was monitored by confocal microscopy at 37°C. Each enlarged vesicle lumen was then photobleached by scanning with a 488 nm wavelength of argon laser at the highest power. Recovery of fluorescence in the lumen was then monitored every 15 s with low laser power after photobleaching. Scale bar, 5 μm. The movie is played at a speed of 75 s/s.